
📑 Table of Contents
🔹The Story of Atoms
Everything around us is made of atoms – the building blocks of matter. But atoms are not indivisible (as once thought). Step by step, scientists discovered electrons, protons, neutrons, and proposed models to explain how these particles are arranged. This journey of Atomic Structure is fascinating and crucial for exams.
🧩 Matter & Atoms
- Matter → made of tiny particles = atoms.
- Atom → made of subatomic particles:
- Protons (+): positive, in nucleus.
- Neutrons (0): neutral, in nucleus.
- Electrons (–): negative, revolve in shells.
💡 Word “Atom” = Greek atomos = indivisible.
💡 In India, Maharshi Kanada introduced “Anu” and “Paramanu”.
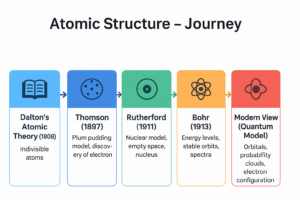
📘 Dalton’s Atomic Theory (1808)
- Atoms are indivisible, cannot be created/destroyed.
- Atoms of same element = identical.
- Atoms of different elements = different.
- Compounds = simple ratios of atoms.
- Chemical reactions = rearrangement of atoms.
⚠️ Limitations:
- Atoms are divisible (discovery of e⁻, p⁺, n).
- Isotopes exist → atoms of same element not identical.
🔬 Discovery of Subatomic Particles
- Electron (–): J.J. Thomson (1897), using cathode rays.
- Proton (+): Eugen Goldstein (1886), using canal rays.
- Neutron (0): James Chadwick (1932).
🏛️ Atomic Models
🍮 1. Thomson’s Model (1897) – Plum Pudding
- Atom = positively charged sphere with electrons embedded (like seeds in watermelon).
- ❌ Failed – couldn’t explain later experiments.
🎯 2. Rutherford’s Model (1911) – Nuclear Model
- Gold foil experiment with α-particles.
- Findings:
- Atom is mostly empty space.
- Small, dense, positively charged nucleus at centre.
- Electrons revolve around nucleus.
- ❌ Couldn’t explain why electrons don’t collapse into nucleus.
🌟 3. Bohr’s Model (1913) – Energy Levels
- Electrons revolve in fixed shells (K, L, M, N …).
- Each shell has fixed energy.
- Electrons absorb/release energy while jumping shells → explains spectra.
- ✔ Solved stability problem.
⚛️ Structure of an Atom
- Nucleus: Protons + Neutrons (nucleons), dense, heavy, +ve charge.
- Extra-nuclear Region: Electrons in shells, occupy volume, mostly empty space.
- Neutral atom: protons = electrons.
📊 Subatomic Particles at a Glance
| Particle | Symbol | Charge | Mass (kg) | Location |
|---|---|---|---|---|
| Electron | e⁻ | –1 | 9.11 × 10⁻³¹ | Outside nucleus |
| Proton | p⁺ | +1 | 1.672 × 10⁻²⁷ | Inside nucleus |
| Neutron | n | 0 | 1.675 × 10⁻²⁷ | Inside nucleus |
🔢 Atomic Number & Mass Number
- Atomic number (Z): No. of protons = No. of electrons.
- Mass number (A): Protons + Neutrons.
- Neutrons = A – Z.
- Symbolic notation: $, ^{A}_{Z}X $.
🔬 Electronic Configuration – Bohr–Bury Rules
- Max electrons in a shell = 2n².
- K = 2, L = 8, M = 18, N = 32.
- Outermost shell → max 8 electrons (except K = 2).
Examples:
- H (Z=1): 1
- He (Z=2): 2
- Li (Z=3): 2,1
- Ne (Z=10): 2,8
- Na (Z=11): 2,8,1
- K (Z=19): 2,8,8,1
📝 Quick Recap: Atomic Structure :
✔ Atoms → smallest unit of matter.
✔ Subatomic particles: p⁺, e⁻, n.
✔ Dalton’s theory → first atomic theory (with limitations).
✔ Thomson → Plum pudding model.
✔ Rutherford → Nuclear model (empty space + nucleus).
✔ Bohr → Energy levels/shells.
✔ Chadwick → Neutron discovery.
✔ Atomic number (Z), Mass number (A), Symbolic notation.
✔ Electronic configuration → Bohr–Bury rules.